Describe How Covalent Compounds Are Named
It involves the transfer of electrons from metal atoms to non-metal atoms. Covalent compounds are compounds formed by two elements that are NONMETAL.

Ionic And Covalent Compound Foldable With Naming Foldables Ionic Covalent Bonding
Tap card to see definition.

. Doesnt use prefixes for naming. They differ from each other due to the bonding type between the atoms that take part in making a molecule compound. Carbon tetrachloride covalent d.
Describe the properties of covalent compounds. Prefixes are used to denote the number of atoms 4. For example for CO the name will be carbon monoxide and the final o of mono is dropped.
Click again to see term. Lithium oxide ionic b. All covalent solids can be categorized into two categories.
Diamond is an example of a crystalline solid and it is. Tap again to see term. The first element is named first using the elements name.
Naming binary two-element covalent compounds is similar to naming simple ionic compounds. The one located in the group with a smaller number gets named first. Describe how to write a formula for a covalent compound Method to deduce the formulae of covalent compoundsNon-metals combine with non-metals to form covalent compounds.
Identify the elements in the chemical formula. If both elements are in the same group the element with the higher period number is written first in the name. It involves the sharing of electrons between atoms of non-metals.
Well use H2O as an example although it is commonly known as water. Table shows the number of electrons needed by an atom of a non-metal to achieve a stable noble gas electron arrangement. Rules for Binary Covalent Compounds.
The second element is named by taking the stem of the element name and adding the suffix -ide. Calcium chloride ionic 5. They are named as multiplying prefixname of first element multiplying prefixname of second element two words If there is only one atom of the first element the multiplying prefix is omitted.
Greek prefixes see the Table provided at the bottom of this page are used to. The first element in the formula is simply listed using the name of the element. How do the name of covalent compounds differ from the names of ionic compounds.
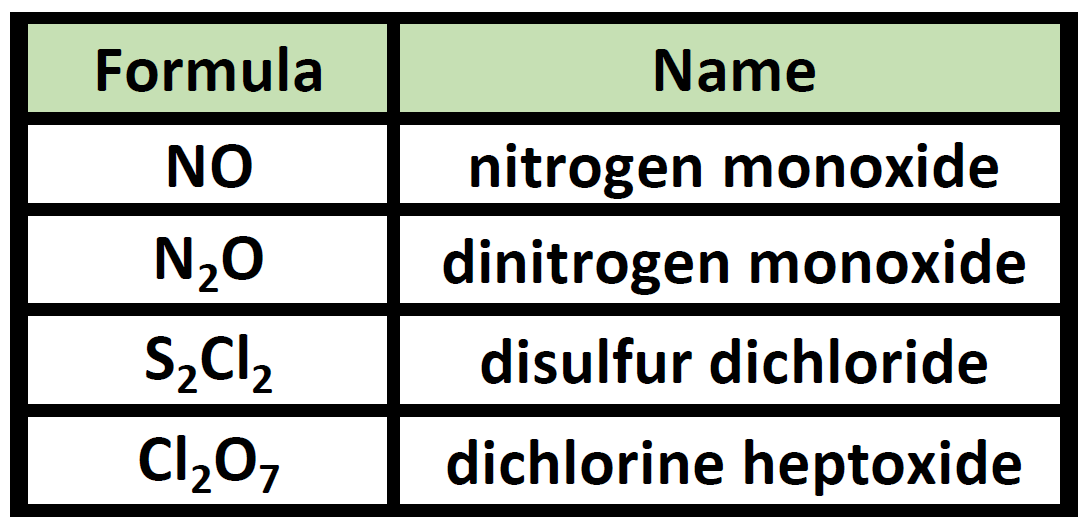
When naming covalent compounds all atoms are nonmetals. 5585 23545 81600 Greek numerical prefixes are used to indicate the number of atoms of a particular element present in a covalent compound. Nitrogen trifluoride covalent e.
Click card to see definition. As their names suggest ionic compounds are made of ionic bonds and covalent compounds are made of covalent bonds. Crystalline solids are hard materials.
Four atoms of the same element will be named with the prefix _____ while the prefix _____ is used to indicate two atoms of the same element. Covalent compounds are molecules formed by non-metals bonded together by sharing electrons. Write the chemical formulas for following compounds.
The second element in the name is named as if it were an anion ie by adding the suffix -ide to the name of the element. It is formed when metal atoms combine with nonmetal atoms. Copyright C by Holt RlnchaJt and Winston.
Covalent compounds are named by first looking at how many atoms the first element in a molecule has. The prefix mono is never used for naming the first element of a compound. Cation means first and anion means last.
Covalent compounds are named by using numerical prefixes to identify the number of atoms in the molecule. A system of numerical prefixes is used to specify the number of atoms in a molecule. The first element in the formula is simply listed using the name of the element.
It is formed when nonmetal atoms combine with non-metal atoms. A metal with a non-metal. Covalent Compounds Describe how covalent bonds form.
Describe how covalent compounds are named. Name and write the formulas for covalent compound. Carbon Dioxide has one carbon atom and two oxygen atoms as identified by the prefix.
Contrast molecular formulas and empirical formulas. The second element is named by taking the stem of the element name and adding the suffix - ide. Binary covalent compounds contain only two elements.
The final o or a of a prefix is often dropped when the element begins with a vowel. Second element is named as an Anion suffix -ide 3. NAMING COVALENT COMPOUNDS.
Name the metal and change the nonmetal ending to -ide. Carbon monoxide covalent c. Covalent compounds are named by using prefixes that give the number of atoms of each element in the compound.
Since the H contributes. Crystalline solids and amorphous solids. The less electronegative element is named first.
How do you know which element to name first. Ionic bonds occur between two species which are electrostatically attracted towards each other whereas covalent. How to Name Binary Covalent Compounds Step 1.
Remember its only the final o or a. The molecules formed by covalent bonds are called covalent molecules. The element located furthest to the left of the periodic table is named first.
For example Carbon Dioxide CO_2 and Carbon Monoxide CO. Nonmetals are all very electronegative and form bonds through the sharing of electrons to satisfy the octet rule. NAMING COVALENT COMPOUNDS.
Covalent compounds possess many similar properties since they share electrons. Naming binary two-element covalent compounds is similar to naming simple ionic compounds.
Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry
What Are The Rules For Naming Ionic And Covalent Compounds Quora


0 Response to "Describe How Covalent Compounds Are Named"
Post a Comment